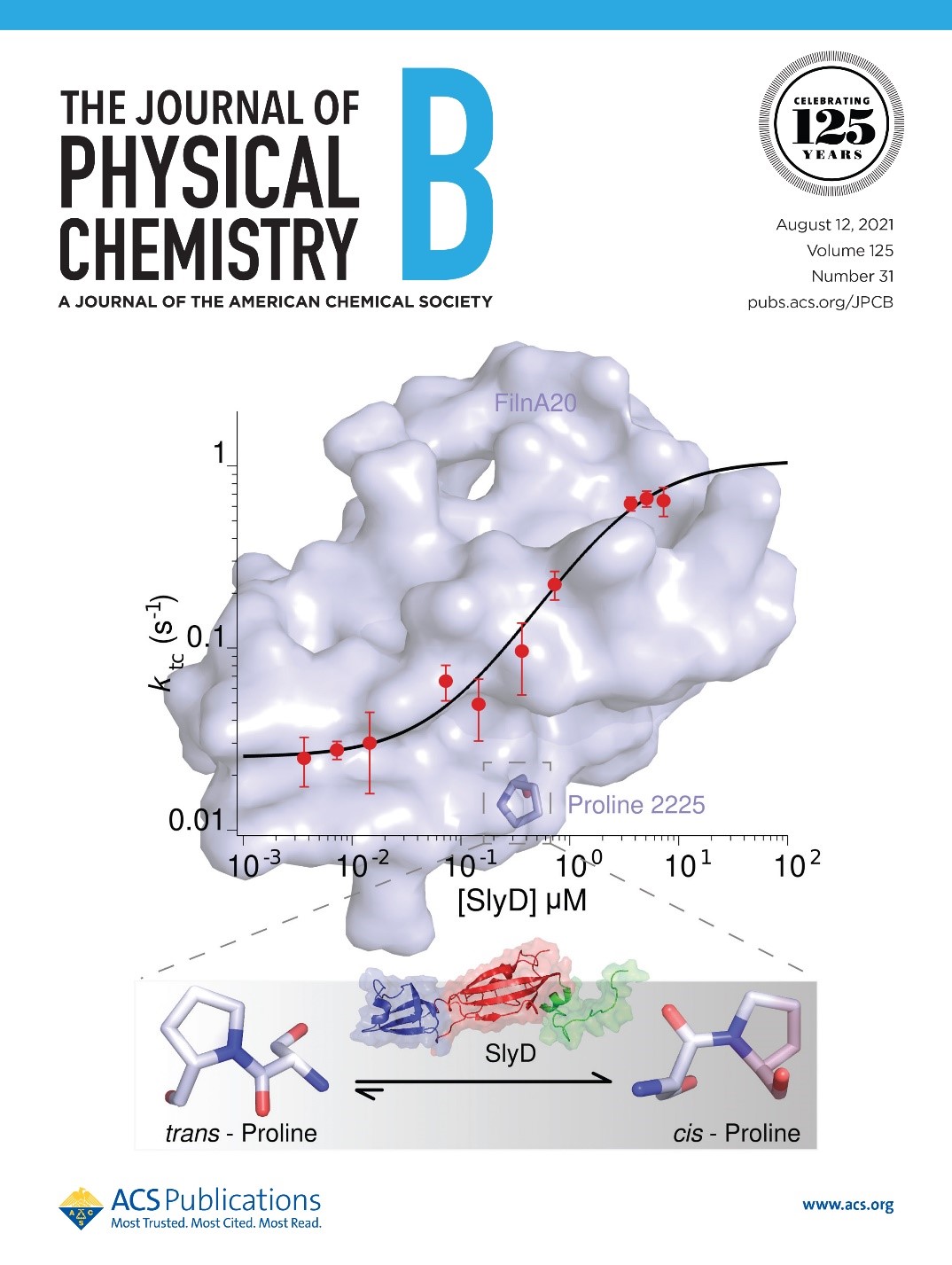
Single-molecule force spectroscopy divulges specific details of how SlyD, a bacterial PPIase, causes a staggering 25-fold acceleration of the trans to cis isomerization of the proline-2225 in an isolated 20th domain of a cytoskeletal mechano-sensing protein, Filamin (FlnA20). SlyD also stabilizes the native-cis state of FlnA20.
Prolyl isomerization is recognized as one of the fundamental regulatory mechanisms, which plays a crucial role in cell signaling, ion channel gating, phage virus infection, and molecular timing. This isomerization is usually slow but often accelerated by an enzyme called peptidyl−prolyl isomerase (PPIase). In the current publication, CasProt researchers at TUM and UPJS used single-molecule force spectroscopy (SMFS) to investigate the impact of a bacterial PPIase, SlyD, on the cis−trans isomerization of the proline 2225 (P2225) in an isolated 20th domain of a cytoskeletal mechanosensing protein filamin-A (FlnA20). To explore the FlnA20−PPIase interaction, they have used multiple SMFS modes, like constant velocity, constant distance, and jumping trap experiments. Their previous study reported the unique nature of the P2225, which is conserved in all naturally occurring filamins and can slowly (minutes) interconvert between cis−trans isomers in the absence of any PPIase. Current results show a staggering 25-fold acceleration of the trans-to-cis isomerization rate in the presence of saturating SlyD concentration (7.25 μM) compared to the nonenzymatic condition. A SlyD concentration-dependent depletion of the trans isomeric lifetime was also observed. Additionally, they observed that SlyD stabilizes the cis-isomer in the native state of FlnA20 by ∼2 kBT. This is the first single-molecule observation of the cis−trans isomerization catalysis by a PPIase in a mechanosensing protein. The findings were published in the Journal of Physical Chemistry B (IF 2.991) (Sengupta A, Rognoni LE, Merkel U, Žoldák G, Rief M. 2021. SlyD Accelerates trans-to-cis Prolyl Isomerization in a Mechanosignaling Protein under Load. J. Phys. Chem. B 2021, 125, 31, 8712–8721.)