As part of the CasProt project, group of Assoc. Prof. Erik Sedlák from the Center for Interdisciplinary Biosciences (CIB) from the Pavol Jozef Šafárik University in Košice developed a matrix for one-step isolation of proteins by affinity chromatography in the CIB laboratories in cooperation with the group of Prof. Andreas Plückthun from the University of Zurich. The basis of the matrix is the protein DARPin (Designed Ankyrin Repetitive Protein) with high affinity for maltose-binding protein (MBP), which was modified by the method of rational design for the needs of affinity chromatography. In this work it was shown not only the high affinity of such a matrix for MBP conjugated proteins, but also the capacity and reproducibility of isolation in this way. This method was published in the International Journal of Biological Macromolecules (IF 6.853) (Nemergut M., Škrabana R., Berta M., Plückthun A., Sedlák E. (2021). Purification of MBP fusion proteins using engineered DARPin affinity matrix. J. Biol. Macromol. 187, 105-112.)
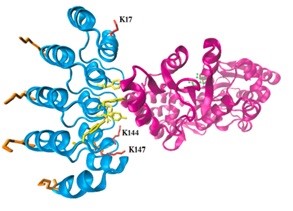 |
The structure of the DARPin-MBP complex, which is the basis of the developed purification method, with marked lysines that have been modified by rational design (PDB: 1SVX and 1ANF). |
 |
|
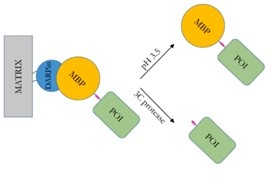
| Schematic representation of MBP-protein isolation by affinity chromatography with DARPin. | |




